Abstract

ProTmune is an ex vivo pharmacologically-modulated, next-generation mobilized peripheral blood (mPB) cell graft with FDA fast track designation for the reduction of incidence and severity of acute graft versus host disease (GvHD) in patients undergoing allogeneic hematopoietic cell transplantation (HCT).
Here we report the 1-year safety and efficacy data from the Phase 1 stage of PROTECT, an ongoing Phase 1 open-label / Phase 2 blinded, randomized and controlled trial of ProTmune in adult subjects with hematologic malignancy undergoing matched unrelated donor HCT following myeloablative conditioning and receiving standard GvHD prophylaxis of methotrexate and tacrolimus.
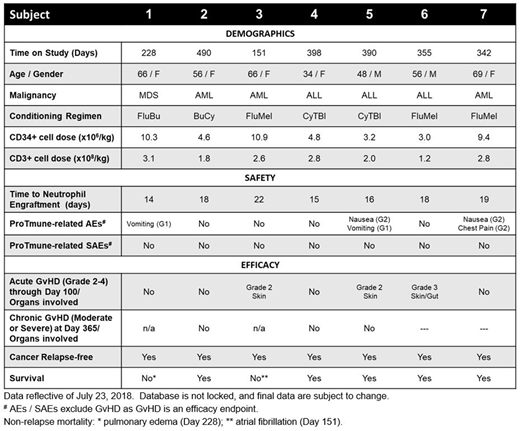
Seven subjects were administered ProTmune in the Phase 1 stage of PROTECT. Underlying hematologic diseases included ALL (n=3), AML (n=3) and MDS (n=1). Subjects were predominantly female (n=5) with a median age of 56 (range 34 to 69) and a median weight of 79 kg (range 53 to 133). As of July 23, 2018, five subjects remain on study with a median follow up of 390 days (range 342 to 490 days).
ProTmune was manufactured on-site on the day of HCT by pharmacologically-modulating a conventional mobilized peripheral blood (mPB) cell graft ex vivo with 16,16-dimethyl prostaglandin E2 and dexamethasone, to enhance the biologic properties and therapeutic function of the graft. All ProTmune units were manufactured successfully with a mean of 6.6 (range 3.0 to 11.0) x106 CD34+ cells/kg and 2.3 (range 1.2 to 3.1) x108 CD3+ cells/kg.
ProTmune was well tolerated. ProTmune-related AEs were all mild to moderate and consisted of nausea, vomiting and non-cardiac chest pain on the day of administration. No ProTmune-related SAEs have been reported. All subjects engrafted with no primary or secondary graft failure. Average time to neutrophil engraftment was 17 days (range 14 to 22).
Day 100 acute GvHD assessment revealed three of the seven subjects had steroid responsive acute CIBMTR Grade 2-4 GvHD with median time of seven days [range 5 to 8 days] to resolution of the maximum GvHD grade.
Since therapeutic approaches intended to reduce acute GvHD may increase disease relapse risk, composite endpoints are being explored by the HCT community to more thoroughly define transplant success. 1-year current GvHD-free, relapse-free survival (CGRFS) is one endpoint under study measuring proportion of subjects that are alive without relapse and without active moderate or severe chronic GvHD per NIH consensus criteria at Day 365.
We intend to present final 1-year CGRFS on the seven Phase 1 subjects at the ASH Annual Meeting. To date, non-relapse mortality, deemed not attributable to ProTmune, occurred in two subjects (Subject 1 on Day 228 from pulmonary edema; Subject 3 on Day 151 from cardiac arrhythmia). There has been no disease relapse, and no moderate / severe chronic GvHD at Day 365. Two subjects are pending the 1-year assessment.
The Phase 2 stage of PROTECT is currently enrolling a planned 60 subjects at 15 U.S. centers. Subjects with hematologic malignancies undergoing matched unrelated donor HCT following myeloablative conditioning are randomized 1:1 to receive in a blinded manner, either ProTmune or a conventional matched unrelated donor mPB unit as the cell graft.
Maziarz:Athersys, Inc.: Patents & Royalties; Incyte: Consultancy, Honoraria; Kite Therapeutics: Honoraria; Juno Therapeutics: Consultancy, Honoraria; Novartis Pharmaceuticals Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Saad:Actinium: Consultancy. Diaz:Fate Therapeutics, Inc.: Employment. Medcalf:Fate Therapeutics, Inc.: Employment. Storgard:Fate Therapeutics, Inc.: Employment, Equity Ownership. Deol:Kite Pharmaceuticals: Consultancy; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal